John Dalton, born on the 6th of Semptember 1766.
He is an english Chemist, metreologist, physicists.
He is well known for modern atomic theory and his research for colorblindness which is now called, daltonisms.
His Influences was john gough.
Atomic Theory:
Thursday, July 8, 2010
Group 9: Que, Recio, Reyes, Sanchez
WHO IS DEMOCRITUS?
Back in ancient Greece, in a place named Abdera, Thace was born a boy named Democritus. His father was a wealthy man indeed. As he grew up, he was highly-esteemed like his fellow citizens. He never really was interested in anything. He was mostly silent and a simple boy. Most of his time was devoted to studying. His friend was Hippocrates. He was the student of Leuppicus. He was called "the Mocker" or the "Laughing Philosopher for he was always joking around. He became blind due to old age, and he died around the age of 90 - 101.
WHO WAS ARISTOTLE?
Aristotle was a boy born in Stageira, Chalcidice. He was trained and educated to be an aristocrat. He was a Philosopher; Plato's student, and Alexander the Great's Adviser. He was an important figure to the Western Philosophy's founding. He had attended Plato's academy in Athens at the age of 18. He was appointed head of Royal aCademy in Macedon.
UNDER CONSTRUCTION.
Back in ancient Greece, in a place named Abdera, Thace was born a boy named Democritus. His father was a wealthy man indeed. As he grew up, he was highly-esteemed like his fellow citizens. He never really was interested in anything. He was mostly silent and a simple boy. Most of his time was devoted to studying. His friend was Hippocrates. He was the student of Leuppicus. He was called "the Mocker" or the "Laughing Philosopher for he was always joking around. He became blind due to old age, and he died around the age of 90 - 101.
WHO WAS ARISTOTLE?
Aristotle was a boy born in Stageira, Chalcidice. He was trained and educated to be an aristocrat. He was a Philosopher; Plato's student, and Alexander the Great's Adviser. He was an important figure to the Western Philosophy's founding. He had attended Plato's academy in Athens at the age of 18. He was appointed head of Royal aCademy in Macedon.
UNDER CONSTRUCTION.
Character Profile of Niels Bohr
Why did he propose this model? And how did he come up with this model?
Bohr contradicted Rutherford`s Nuclear Model of the atom and started experimenting on how protons, electrons and neutrons are arranged in an atom. He invented a device that made these move together. In this experiment, he discovered that electrons orbit around the nucleus which contains the protons and neutrons.
When and What did he conduct in his experiment?
joseph louis proust
joseph louis proust
french chemist who was born in angers,france on september 26 1754, and died ion 1826
necame known abpout his reaserch work on thye stedinness of compopsition of chemical compounds
he analytically studied the two tin oxide and two iron sulfides
proving that had different composition and that there were no substances with intremidiate composition
his labratorial tests showed yhat the variable composition oxides
studied by berthellot were hydrated products
he also performed series of researches to characterize different tylpes of sugars
present in vegetable product
GRP.10
Danna Santos
Maia Tangco
Milana Tapawan
Ji u Yoon (Diana)
french chemist who was born in angers,france on september 26 1754, and died ion 1826
necame known abpout his reaserch work on thye stedinness of compopsition of chemical compounds
he analytically studied the two tin oxide and two iron sulfides
proving that had different composition and that there were no substances with intremidiate composition
his labratorial tests showed yhat the variable composition oxides
studied by berthellot were hydrated products
he also performed series of researches to characterize different tylpes of sugars
present in vegetable product
GRP.10
Danna Santos
Maia Tangco
Milana Tapawan
Ji u Yoon (Diana)
Lavoisier, Antoine (1743-1794)
 Antoine-Laurent de Lavoisier (also Antoine Lavoisier after the French Revolution; (26 August 1743 – 8 May 1794); (French pronunciation: [ɑ̃twan lɔʁɑ̃ də lavwazje]), the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology. He stated the first version of the law of conservation of mass, recognized and named oxygen (1778) and hydrogen (1783), abolished the phlogiston theory, helped construct the metric system, wrote the first extensive list of elements, and helped to reform chemical nomenclature. He discovered that, although matter may change its form or shape, its mass always remains the same.
Antoine-Laurent de Lavoisier (also Antoine Lavoisier after the French Revolution; (26 August 1743 – 8 May 1794); (French pronunciation: [ɑ̃twan lɔʁɑ̃ də lavwazje]), the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology. He stated the first version of the law of conservation of mass, recognized and named oxygen (1778) and hydrogen (1783), abolished the phlogiston theory, helped construct the metric system, wrote the first extensive list of elements, and helped to reform chemical nomenclature. He discovered that, although matter may change its form or shape, its mass always remains the same. Over the 20 year period 1770 - 1790, the science of chemistry experienced a revolution so fundamental and so complete that there has been nothing like it since. The architect of the revolution was one man, Antoine Lavoisier. Lavoisier believed that weight was conserved through the course of chemical reactions even those involving gases. He explained combustion (and respiration) in terms of chemical reactions that involve a component of air which he called oxygen. His venue for the chemical revolution came in 1775, when he was appointed Commissioner of the Royal Gunpowder and Saltpeter Administration. As such, he was able to build a fine laboratory at the Paris Arsenal and make important connections to the scientific community of all of Europe. One of the first chemists to adopt Lavoisier's theories was Joseph Black who taught them as early as 1784.
Contributions to chemistry
Lavoisier demonstrated the role of oxygen in the rusting of metal, as well as oxygen's role in animal and plant respiration. Working with Pierre-Simon Laplace, Lavoisier conducted experiments that showed that respiration was essentially a slow combustion of organic material using inhaled oxygen. Lavoisier's explanation of combustion disproved the phlogiston theory, which postulated that materials released a substance called phlogiston when they burned.
GROUP 3
Constantino, Leslie
Cruz, Xyrill
Darocca, Tish
Dayo, Monica
John Dalton and His Atomic Model (Group 4) Kristine Gadia, Shenen Gazmen, Grazie De Guzman and Roeckl Desingano
John Dalton
Born: September 6 1766
Birthplace: Eaglesfield, Cumber, England
Death: July 27 1844
At the age of 15 he joined his brother Jonathan in running a Quaker's school. At the age of 24 he have decided to take up Law or Medicine but his family was not there to give him the support for his works. At 1793, he moved to Manchester. Through John Gough, a blind philosopher and polymath Dalton was able to have the knowledge about Science. He became a Mathematics and Natural Philosophist teacher at Manchester. And he became a private tutor at the said subjects. Dalton's early life was highly influenced by a prominent Eaglesfield Quaker named Elihu Robinson, a competent meteorologist and instrument maker, who got him interested in problems of mathematics and meteorology. Dalton contributed solutions of problems at different subjects during his times at Kendal. From 1787, he was able to enter 200,000 observation in his "meteological diary" for the past 57 years. He was able to publish his first writing, The Meteorological Observations and Essay in the year 1793.
Later Years
 Dalton communicated his atomic theory to Thompson. Included an outline of it in the 3rd edition of the System Of Chemisty. He gave futher look to the first volume of his New System of Chemical Philisophy. The first part of the second colume did not appear till 1827. It was not explained by the care of preparation. The second part of vol. ii did not also appear.
Dalton communicated his atomic theory to Thompson. Included an outline of it in the 3rd edition of the System Of Chemisty. He gave futher look to the first volume of his New System of Chemical Philisophy. The first part of the second colume did not appear till 1827. It was not explained by the care of preparation. The second part of vol. ii did not also appear.
His discovered was regarded by his self in importance to the atomic theory.
James Prescott Joule was one of his famous pupil.
John Dalton's atomic theory
John Dalton's theory says that the different elements could be based on the different atomic weight.. He stated his theory in a lecture to the Royal Institution in 1803.
Dalton's five main points in his atomic theory:
1.All matter is composed of atoms
2.Atoms cannot be made or destroyed
3.All atoms of the same element are identical
4.Different elements have different types of atoms
5.Chemical reactions occur when atoms are rearranged
6.Compounds are formed from atoms of the constituent elements.
Using his theory, Dalton explained numerous theories but his computation is not that accurate because he gave oxygen an atomic weight of 7 instead of 8. Despite these errors, Dalton's theory provided a logical explanation of concepts, and led the way into new fields of experimentation.
Reference: http://en.wikipedia.org/wiki/John_Dalton#Atomic_theory
http://www.rsc.org/chemsoc/timeline//pages/1803.html
Born: September 6 1766
Birthplace: Eaglesfield, Cumber, England
Death: July 27 1844
At the age of 15 he joined his brother Jonathan in running a Quaker's school. At the age of 24 he have decided to take up Law or Medicine but his family was not there to give him the support for his works. At 1793, he moved to Manchester. Through John Gough, a blind philosopher and polymath Dalton was able to have the knowledge about Science. He became a Mathematics and Natural Philosophist teacher at Manchester. And he became a private tutor at the said subjects. Dalton's early life was highly influenced by a prominent Eaglesfield Quaker named Elihu Robinson, a competent meteorologist and instrument maker, who got him interested in problems of mathematics and meteorology. Dalton contributed solutions of problems at different subjects during his times at Kendal. From 1787, he was able to enter 200,000 observation in his "meteological diary" for the past 57 years. He was able to publish his first writing, The Meteorological Observations and Essay in the year 1793.
Later Years
 Dalton communicated his atomic theory to Thompson. Included an outline of it in the 3rd edition of the System Of Chemisty. He gave futher look to the first volume of his New System of Chemical Philisophy. The first part of the second colume did not appear till 1827. It was not explained by the care of preparation. The second part of vol. ii did not also appear.
Dalton communicated his atomic theory to Thompson. Included an outline of it in the 3rd edition of the System Of Chemisty. He gave futher look to the first volume of his New System of Chemical Philisophy. The first part of the second colume did not appear till 1827. It was not explained by the care of preparation. The second part of vol. ii did not also appear.He was the the President of the Lit and Phil until his death. In 1814, he explains the principles of the volumetric analysis. He was one of the earliest workers. in 1840, he was refused by the Royal Society. instead, he published it himself.
His discovered was regarded by his self in importance to the atomic theory.
James Prescott Joule was one of his famous pupil.
John Dalton's atomic theory
John Dalton's theory says that the different elements could be based on the different atomic weight.. He stated his theory in a lecture to the Royal Institution in 1803.
Dalton's five main points in his atomic theory:
1.All matter is composed of atoms
2.Atoms cannot be made or destroyed
3.All atoms of the same element are identical
4.Different elements have different types of atoms
5.Chemical reactions occur when atoms are rearranged
6.Compounds are formed from atoms of the constituent elements.
Using his theory, Dalton explained numerous theories but his computation is not that accurate because he gave oxygen an atomic weight of 7 instead of 8. Despite these errors, Dalton's theory provided a logical explanation of concepts, and led the way into new fields of experimentation.
Reference: http://en.wikipedia.org/wiki/John_Dalton#Atomic_theory
http://www.rsc.org/chemsoc/timeline//pages/1803.html
GROUP3 Xyrill Cruz
Monica Dayo
Leslie Constantino
Tish Darroca
 The most important chemical reaction that he performed was decomposition of the red oxide of mercury to form metallic mercury and the gas he named oxygen. He was the first to weigh all the substances present before and after the reaction. On realization from his quantitative experiments was that when coal was burned, it united with oxygen to form carbon dioxide. Therefore, he concluded that respiration was related to combustion. He found that matter was conserved and its amount remained constant. Because of his findings, this lead to the Law of Conservation of Mass. This law states that matter is neither created nor destroyed during a chemical change. The total mass of the reaction products is always equal to the total mass of the reactants.
The most important chemical reaction that he performed was decomposition of the red oxide of mercury to form metallic mercury and the gas he named oxygen. He was the first to weigh all the substances present before and after the reaction. On realization from his quantitative experiments was that when coal was burned, it united with oxygen to form carbon dioxide. Therefore, he concluded that respiration was related to combustion. He found that matter was conserved and its amount remained constant. Because of his findings, this lead to the Law of Conservation of Mass. This law states that matter is neither created nor destroyed during a chemical change. The total mass of the reaction products is always equal to the total mass of the reactants.
Antoine-Laurent Lavoisier conducts an experiment on human respiration in this drawing made by his wife, who depicted herself at the table on the far right. Courtesy Edgar Fahs Smith Memorial Collection, Department of Special Collections, University of Pennsylvania Library.
Lavoisier investigated the composition of water and air, which at the time were considered elements. He determined that the components of water were oxygen and hydrogen, and that air was a mixture of gases, primarily nitrogen and oxygen. With the French chemists Claude-Louis Berthollet, Antoine Fourcroy and Guyton de Morveau, Lavoisier devised a systematic chemical nomenclature. He described it in Méthode de nomenclature chimique (Method of Chemical Nomenclature, 1787). This system facilitated communication of discoveries between chemists of different backgrounds and is still largely in use today, including names such as sulfuric acid, sulfates, and sulfites.
Pioneer of stoichiometry
LEGACY
 Lavoisier's fundamental contributions to chemistry were a result of a conscious effort to fit all experiments into the framework of a single theory. He established the consistent use of the chemical balance, used oxygen to overthrow the phlogiston theory, and developed a new system of chemical nomenclature which held that oxygen was an essential constituent of all acids (which later turned out to be erroneous). Lavoisier also did early research in physical chemistry and thermodynamics in joint experiments with Laplace. They used a calorimeter to estimate the heat evolved per unit of carbon dioxide produced, eventually finding the same ratio for a flame and animals, indicating that animals produced energy by a type of combustion reaction
Lavoisier's fundamental contributions to chemistry were a result of a conscious effort to fit all experiments into the framework of a single theory. He established the consistent use of the chemical balance, used oxygen to overthrow the phlogiston theory, and developed a new system of chemical nomenclature which held that oxygen was an essential constituent of all acids (which later turned out to be erroneous). Lavoisier also did early research in physical chemistry and thermodynamics in joint experiments with Laplace. They used a calorimeter to estimate the heat evolved per unit of carbon dioxide produced, eventually finding the same ratio for a flame and animals, indicating that animals produced energy by a type of combustion reaction
 However, much to his professional detriment, Lavoisier discovered no new substances, devised no really novel apparatus, and worked out no improved methods of preparation. He was essentially a theorist, and his great merit lay in the capacity of taking over experimental work that others had carried out—without always adequately recognizing their claims—and by a rigorous logical procedure, reinforced by his own quantitative experiments, of expounding the true explanation of the results. He completed the work of Black, Priestley and Cavendish, and gave a correct explanation of their experiments.
However, much to his professional detriment, Lavoisier discovered no new substances, devised no really novel apparatus, and worked out no improved methods of preparation. He was essentially a theorist, and his great merit lay in the capacity of taking over experimental work that others had carried out—without always adequately recognizing their claims—and by a rigorous logical procedure, reinforced by his own quantitative experiments, of expounding the true explanation of the results. He completed the work of Black, Priestley and Cavendish, and gave a correct explanation of their experiments. Detail of picture of a combustion experiment
Detail of picture of a combustion experiment
Monica Dayo
Leslie Constantino
Tish Darroca
Born: August 26, 1743
Birthplace: Paris, France
Died: May 8, 1794
He is known as the Father of Modern Chemistry. His real interest, however, was in science, which he pursued with passion while leading a full public life. On the basis of his earliest scientific work, mostly in geology, he was elected in 1768—at the early age of 25—to the Academy of Sciences, France's most elite scientific society. In the same year he bought into the Ferme Générale, the private corporation that collected taxes for the Crown on a profit-and-loss basis. A few years later he married the daughter of another tax farmer, Marie-Anne Pierrette Paulze, who was not quite 14 at the time. Madame Lavoisier prepared herself to be her husband's scientific collaborator by learning English to translate the work of British chemists like Joseph Priestley and by studying art and engraving to illustrate Antoine-Laurent's scientific experiments.
 The most important chemical reaction that he performed was decomposition of the red oxide of mercury to form metallic mercury and the gas he named oxygen. He was the first to weigh all the substances present before and after the reaction. On realization from his quantitative experiments was that when coal was burned, it united with oxygen to form carbon dioxide. Therefore, he concluded that respiration was related to combustion. He found that matter was conserved and its amount remained constant. Because of his findings, this lead to the Law of Conservation of Mass. This law states that matter is neither created nor destroyed during a chemical change. The total mass of the reaction products is always equal to the total mass of the reactants.
The most important chemical reaction that he performed was decomposition of the red oxide of mercury to form metallic mercury and the gas he named oxygen. He was the first to weigh all the substances present before and after the reaction. On realization from his quantitative experiments was that when coal was burned, it united with oxygen to form carbon dioxide. Therefore, he concluded that respiration was related to combustion. He found that matter was conserved and its amount remained constant. Because of his findings, this lead to the Law of Conservation of Mass. This law states that matter is neither created nor destroyed during a chemical change. The total mass of the reaction products is always equal to the total mass of the reactants. - Antoine Lavoisier, a French chemist, also discovered oxygen in 1775, was the first to recognize it as an element, and coined its name "oxygen" - which comes from a Greek word that means “acid-former”.
Antoine-Laurent Lavoisier conducts an experiment on human respiration in this drawing made by his wife, who depicted herself at the table on the far right. Courtesy Edgar Fahs Smith Memorial Collection, Department of Special Collections, University of Pennsylvania Library.
Analytical chemistry and chemical nomenclature
Lavoisier investigated the composition of water and air, which at the time were considered elements. He determined that the components of water were oxygen and hydrogen, and that air was a mixture of gases, primarily nitrogen and oxygen. With the French chemists Claude-Louis Berthollet, Antoine Fourcroy and Guyton de Morveau, Lavoisier devised a systematic chemical nomenclature. He described it in Méthode de nomenclature chimique (Method of Chemical Nomenclature, 1787). This system facilitated communication of discoveries between chemists of different backgrounds and is still largely in use today, including names such as sulfuric acid, sulfates, and sulfites.
Pioneer of stoichiometry
Laboratory equipment used by Lavoisier circa 1780s
Lavoisier's researches included some of the first truly quantitative chemical experiments. He carefully weighed the reactants and products in a chemical reaction, which was a crucial step in the advancement of chemistry. He showed that, although matter can change its state in a chemical reaction, the total mass of matter is the same at the end as at the beginning of every chemical change. Thus, for instance, if water is heated to steam, if salt is dissolved in water or if a piece of wood is burned to ashes, the total mass remains unchanged. His experiments supported the law of conservation of mass, which Lavoisier was the first to state,[2] although Mikhail Lomonosov (1711–1765) had previously expressed similar ideas in 1748 and proved them in experiments. Others who anticipated the work of Lavoisier include Joseph Black (1728–1799), Henry Cavendish (1731–1810), and Jean Rey (1583–1645).
The work of Lavoisier was translated in Japan in the 1840s, through the process of Rangaku. Page from Udagawa Yōan's 1840 Seimi Kaisō
LEGACY
 Lavoisier's fundamental contributions to chemistry were a result of a conscious effort to fit all experiments into the framework of a single theory. He established the consistent use of the chemical balance, used oxygen to overthrow the phlogiston theory, and developed a new system of chemical nomenclature which held that oxygen was an essential constituent of all acids (which later turned out to be erroneous). Lavoisier also did early research in physical chemistry and thermodynamics in joint experiments with Laplace. They used a calorimeter to estimate the heat evolved per unit of carbon dioxide produced, eventually finding the same ratio for a flame and animals, indicating that animals produced energy by a type of combustion reaction
Lavoisier's fundamental contributions to chemistry were a result of a conscious effort to fit all experiments into the framework of a single theory. He established the consistent use of the chemical balance, used oxygen to overthrow the phlogiston theory, and developed a new system of chemical nomenclature which held that oxygen was an essential constituent of all acids (which later turned out to be erroneous). Lavoisier also did early research in physical chemistry and thermodynamics in joint experiments with Laplace. They used a calorimeter to estimate the heat evolved per unit of carbon dioxide produced, eventually finding the same ratio for a flame and animals, indicating that animals produced energy by a type of combustion reactionLavoisier also contributed to early ideas on composition and chemical changes by stating the radical theory, believing that radicals, which function as a single group in a chemical process, combine with oxygen in reactions. He also introduced the possibility of allotropy in chemical elements when he discovered that diamond is a crystalline form of carbon.
 However, much to his professional detriment, Lavoisier discovered no new substances, devised no really novel apparatus, and worked out no improved methods of preparation. He was essentially a theorist, and his great merit lay in the capacity of taking over experimental work that others had carried out—without always adequately recognizing their claims—and by a rigorous logical procedure, reinforced by his own quantitative experiments, of expounding the true explanation of the results. He completed the work of Black, Priestley and Cavendish, and gave a correct explanation of their experiments.
However, much to his professional detriment, Lavoisier discovered no new substances, devised no really novel apparatus, and worked out no improved methods of preparation. He was essentially a theorist, and his great merit lay in the capacity of taking over experimental work that others had carried out—without always adequately recognizing their claims—and by a rigorous logical procedure, reinforced by his own quantitative experiments, of expounding the true explanation of the results. He completed the work of Black, Priestley and Cavendish, and gave a correct explanation of their experiments. Detail of picture of a combustion experiment
Detail of picture of a combustion experimentOverall, his contributions are considered the most important in advancing chemistry to the level reached in physics and mathematics during the 18th century.[8]
- Constant pressure calorimeter , engraving made by madame Lavoisier for thermochemistry experiments
II-3 Group 2: Joseph Louis Proust
Born: September 26,1754 in Angers, France
Died: July 5,1826 Paris, France
Occupation: French Chemist
Biography
His Father served as an apthecary in Angers.Joseph studied chemistry in his father's shop and later came to Paris where he gained the appointment of apothecary in chief to the Salpetriere.
Under Carlos IV's influence Proust went to Spain. There he taught at the Chemisrty School in Segovia and at the University of Salamanca. But when Napoleon invaded Spain, they burned Proust's laboratory and forced him back to France.
In 1785 Proust accepted a lucrative teaching position offered by the Spanish government. He spent the next twenty years in Spain at various posts in Madrid and Segovia, thus missing the French Revolution and the rise to power of Napoleon Bonaparte (1769-1821). In addition to teaching chemistry, Proust worked for the Spanish government, frequently conducting geological surveys and analyzing the nation's mineral resources.
Proust’s largest accomplishment into the realm of science was disproving Berthollet with the law of definite proportions, which is sometimes also known as Proust's Law. Proust studied copper carbonate, the two tin oxides,and the two iron sulfides to prove this law. He did this by making artificial copper carbonate and comparing it to natural copper carbonate Between the two types of the other compounds, Proust showed that no intermediate indeterminate compounds exist between them. Proust published this paper in 1794, but the law was not accepted until 1811, when the Swedish chemist Jöns Jacob Berzelius gave him credit for it.
Atomic Theory
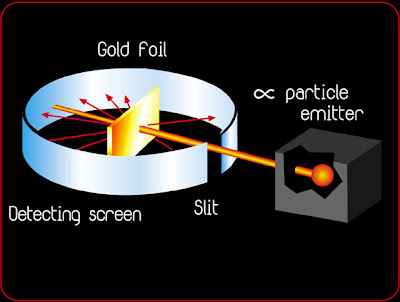
The gold foil experiment
Top: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Bottom: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus
Law of Definite Proportion
In chemistry, the law of definite proportions and also the elements, sometimes called Proust's Law, states that a chemical compound always contains exactly the same proportion of elements by mass. An equivalent statement is the law of constant composition, which states that all samples of a given chemical compound have the same elemental composition.
History
This observation was first made by the French chemist Joseph Proust based on several experiments conducted between 1798 and 1804. Based on such observations, Proust made statements like this one, in 1806:
"I shall conclude by deducing from these experiments the principle I have established at the commencement of this memoir, viz. that iron like many other metals is subject to the law of nature which presides at every true combination, that is to say, that it unites with two constant proportions of oxygen. In this respect it does not differ from tin, mercury, and lead, and, in a word, almost every known combustible."
The law of definite proportions might seem obvious to the modern chemist, inherent in the very definition of a chemical compound. At the end of the 18th century. The first proposed, it was a controversial statement and was opposed by other chemists, most notably Proust's fellow Frenchman Claude Louis Berthollet, who argued that the elements could combine in any proportion. The very existence of this debate underscores that at the time, the distinction between pure chemical compounds and mixtures had not yet been fully developed. The law of definite proportions contributed to, and was placed on a firm theoretical basis by, the atomic theory that John Dalton promoted beginning in 1803, which explained matter as consisting of discrete atoms, that there was one type of atom for each element, and that the compounds were made of combinations of different types of atoms in fixed proportions
A related early idea was Prout's hypothesis, which supposed that hydrogen was the only functional unit, and was related to the whole number rule, which was the rule of thumb that atomic masses were whole number multiples of the mass of hydrogen. This was later rejected in the 1820s and 30s following more refined measurements of atomic mass, notably by Jöns Jacob Berzelius, which revealed in particular that the atomic mass of chlorine was 35.45, which was incompatible with the hypothesis.
By: Arcilla, Atis, Bongato, Valenzuela
How Atoms Were Discovered: Group 3
Progression of Atoms
by Group 3
 DEMOCRITUS (460-370 B.C) Democritus expanded the idea to state that matter was composed of small particles called "atoms" that could be divided no further.These atoms were all composed of the same primary matter with the only differences between them being their size, shape and weight. The differences in these characteristics explained the differences in the properties of the matter around us.
DEMOCRITUS (460-370 B.C) Democritus expanded the idea to state that matter was composed of small particles called "atoms" that could be divided no further.These atoms were all composed of the same primary matter with the only differences between them being their size, shape and weight. The differences in these characteristics explained the differences in the properties of the matter around us.  JOHN DALTON (1786-1844) During the 19th century, a vast amount of data on how substances react with each other was collected. From this data, some simple laws of chemical reactivity had been devised. Dalton's theory can be summarized as follows:
JOHN DALTON (1786-1844) During the 19th century, a vast amount of data on how substances react with each other was collected. From this data, some simple laws of chemical reactivity had been devised. Dalton's theory can be summarized as follows: - Matter is composed of small particles called atoms
- 1. All atoms of an element are identcial, but are different from those of any other element.
- During chemical reactions, atoms are neither created nor destroyed, but are simply rearranged.
- Atoms always combine in whole number multiples of each other
 Dmitri Mendeleev (1834 - 1907) Dmitri Mendeleev attempted to classify the elements not by some "accidental, or instinctive reasons, but by some exact principle. The only unchanging numerical data available at this time was the atomic weight. By arranging the elements in order of increasing atomic weight he discovered that there existed a periodicity of the elemental properties. He used this periodicity to create a table in which that elements with similar properties were vertically aligned with each other.
Dmitri Mendeleev (1834 - 1907) Dmitri Mendeleev attempted to classify the elements not by some "accidental, or instinctive reasons, but by some exact principle. The only unchanging numerical data available at this time was the atomic weight. By arranging the elements in order of increasing atomic weight he discovered that there existed a periodicity of the elemental properties. He used this periodicity to create a table in which that elements with similar properties were vertically aligned with each other. J.J THOMPSON(1856-1940) At approximately the same time as radioactivity was being investigated, J.J. Thomson and others were performing experiments with cathode ray tubes.
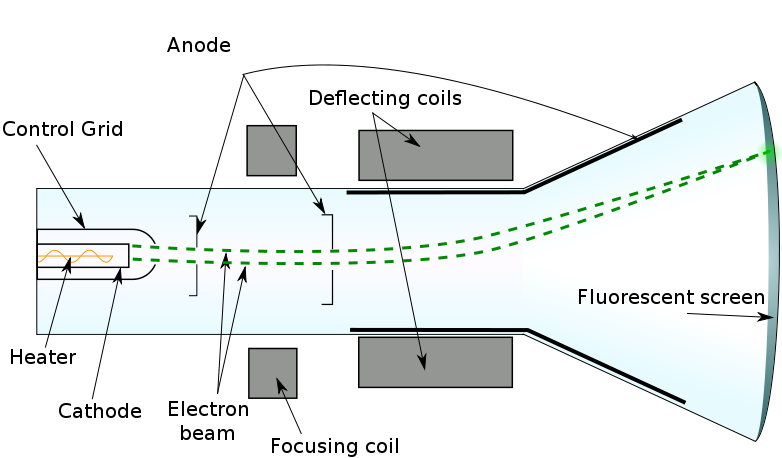
J.J THOMPSON(1856-1940) At approximately the same time as radioactivity was being investigated, J.J. Thomson and others were performing experiments with cathode ray tubes.  Cathode Ray tube - It is an evacuated tube that contains a small amount of gas between two metallic plates. When a potential is placed between the cathode (the negatively charged plate) and the anode (the positively charged plate) a "ray" of electric current passes from one plate to the other.
Cathode Ray tube - It is an evacuated tube that contains a small amount of gas between two metallic plates. When a potential is placed between the cathode (the negatively charged plate) and the anode (the positively charged plate) a "ray" of electric current passes from one plate to the other. Thomson discovered that this ray was actually composed of particles. Thomson proposed that an atom was composed of a spherical ball of positive charge with "corpuscles" of negative charge imbedded in it. The corpuscles would later become known as electrons.
This is how to represent an atom
In 1909, Rutherford set a fellow scientist, Hans Geiger, and a student, Ernest Marsden, to work on this problem. They devised a system that allowed alpha particles (the nuclei of helium atoms) to be shot at a very thin piece of gold foil and the trajectory of the particles monitored.
In order to account for the fact that many of the alpha particles passed through the gold film, Rutherford discounted Thompson's solid ball model of the atom, and believed that the central positive charge of the atom represented only a small fraction of the atom's size, and that the remainder was primarily empty space. He calculated that, while an individual atom was about 1x10-10 meters in diameter, the nuclear diameter was only about 1x10-14 meters.
Robert Millikan (1868 - 1953)
It was not until the work of Robert Millikin that the number value of this charge could be determined.
Millikin was able to calculate the charge on each of the droplets he tested. The calculated charges on the droplets all turned out to multiples of a single number. Millikin therefore reasoned the elementary charge, or the smallest of charge, must be equal to this value. By combining his new information with the mass to charge ratio for the electron determined by Thomson, the mass of an electron was calculated for the first time.
SUMMARY ABOUT ATOMS:
- Atoms are composed of three elementary particles: the electron, the proton and the neutron.
- Most of the mass of the atom is concentrated in the nucleus of the atom. The protons and neutrons reside in the nucleus while the electrons exist outside of the nucleus.
- The number of protons is equal to the number of electrons.
- The type of element each atom is determined by the number of protons it has.
- The number of protons in an element is equal to the atomic number.
- Atoms do not have the same atomic mass. Atoms of the same element with different masses are called isotopes.
- The sum of the number of protons and neutrons in a particular atom is called the mass number. The mass number is different for different isotopes of the same element.
Members:
Leslie Constantino
Xyrill Cruz
Tish Darroca
Monica Dayo
Leslie Constantino
Xyrill Cruz
Tish Darroca
Monica Dayo
Group 9 room 3 Biography:)
DEMOCRITUS:
Democritus was an ancient Greek philosopher born in Abdera, Thace, Greece.
-died at the age of 90-101.
-was an influential pre-Socratic philisopher.
-formulated the atomic theory for the cosmos.
His father is very wealthy. He is friends with Hippocrates.
He was highly-esteemed with his fellow citizens.He is can often be describes as disinterested, simple modest and lived exclusively for his studies. One story tells of him blinding himself so he could be less disturbed. But, it may also be because of his old age that he lost his sight.
His teacher is Leucippus.
Also known as the 'Laughing Philosopher" because of his cheerful personality. To his fellow citizens, he is also known as 'The Mocker".
Aristotle:
Aristotle was a Greek philosopher, a student of Plato and teacher of Alexander the Great.
-born in Stageira, Chalcidice
-died aged 61-62
-one of the most important founding figures in Western Philosophy.
-was trained and educated to be an aristocrat
-went to Plato's Academy in Athens at the age of 18
-was appointed head of royal academy in Macedon.
-taught Ptolemy, Alexander and Cassander
-He had his own school ( named Lyceum)
-people thought he was involved in the death of Alexander
Group 9: Martha Que, Pauline Recio, Honeylette Sanchez, Celina Reyes
Democritus was an ancient Greek philosopher born in Abdera, Thace, Greece.
-died at the age of 90-101.
-was an influential pre-Socratic philisopher.
-formulated the atomic theory for the cosmos.
His father is very wealthy. He is friends with Hippocrates.
He was highly-esteemed with his fellow citizens.He is can often be describes as disinterested, simple modest and lived exclusively for his studies. One story tells of him blinding himself so he could be less disturbed. But, it may also be because of his old age that he lost his sight.
His teacher is Leucippus.
Also known as the 'Laughing Philosopher" because of his cheerful personality. To his fellow citizens, he is also known as 'The Mocker".
Aristotle:
Aristotle was a Greek philosopher, a student of Plato and teacher of Alexander the Great.
-born in Stageira, Chalcidice
-died aged 61-62
-one of the most important founding figures in Western Philosophy.
-was trained and educated to be an aristocrat
-went to Plato's Academy in Athens at the age of 18
-was appointed head of royal academy in Macedon.
-taught Ptolemy, Alexander and Cassander
-He had his own school ( named Lyceum)
-people thought he was involved in the death of Alexander
Group 9: Martha Que, Pauline Recio, Honeylette Sanchez, Celina Reyes
Democritus and Aristotle (group 1)
The Billard Ball Model
-Was the first model of an atom. Described as indivisible.
Democritus- (ca. 460 BCE- ca. 370 BCE) was an ancient greek philosopher born in Abdera, Thrace, Greece. he is a pupil of Leucippus, the man who discovered the existence of an atom. He proposed the Billiard Ball Model which represents an atom which was characterised as indivisible.Aristotle- (384 BC – 322 BC) was a Greek philosopher, a student of Plato and teacher of Alexander the Great.
Group 9 - Historian
THE HISTORICA FILES:
BASIC FACTUAL BACKGROUND OF THE CENTURY:
- Four Questions:
xoxoxoWhere does everything come from?
xoxoxoWhat made everything?
xoxoxoHow do we describe nature mathemtaically?
xoxoxoHow do we explain the plurality of things found in nature?
-Western Philosphy Began in the 6th Century BCE in Ancient Greece.
- Everyone was trying to understand the very basic essence of the world.
- Everything was rational; fiction and myth was rejected immediately.
MILESIAN SCHOOL:
- The first Philosophers came from Miletus.
- Thales came up with the conclusion that water was the element where everything had come from.
PYTHAGOREANISM:
- Pythagoras made this philosophy.
- It was aimed at numbers, but also telling others about a harmonious life.
EPHESIAN SCHOOL:
- Heraclitus thought that the primary substance is fire. It begins in fire and ends in fire.
ELEATIC SCHOOL:
- Xenophanes declared God as the central form of eternal unity.
PLURALIST SCHOOL:
- Empedocles made the philosophy where in all elements are important to human kind but he added that of love as unity and strife as separation.
ATOMIST SCHOOL:
- Leucippus was the first to make the materialistic ways; Democritus was his student.
SOPHISM:
- Apprehension of the senses is their basis.
BASIC FACTUAL BACKGROUND OF THE CENTURY:
- Four Questions:
xoxoxoWhere does everything come from?
xoxoxoWhat made everything?
xoxoxoHow do we describe nature mathemtaically?
xoxoxoHow do we explain the plurality of things found in nature?
-Western Philosphy Began in the 6th Century BCE in Ancient Greece.
- Everyone was trying to understand the very basic essence of the world.
- Everything was rational; fiction and myth was rejected immediately.
MILESIAN SCHOOL:
- The first Philosophers came from Miletus.
- Thales came up with the conclusion that water was the element where everything had come from.
PYTHAGOREANISM:
- Pythagoras made this philosophy.
- It was aimed at numbers, but also telling others about a harmonious life.
EPHESIAN SCHOOL:
- Heraclitus thought that the primary substance is fire. It begins in fire and ends in fire.
ELEATIC SCHOOL:
- Xenophanes declared God as the central form of eternal unity.
PLURALIST SCHOOL:
- Empedocles made the philosophy where in all elements are important to human kind but he added that of love as unity and strife as separation.
ATOMIST SCHOOL:
- Leucippus was the first to make the materialistic ways; Democritus was his student.
SOPHISM:
- Apprehension of the senses is their basis.
Ernest Rutherford - Group 6
Ernest Rutherford
Biography
Ernest Rutherford was the son of James Rutherford, a farmer, and his wife Martha Thompson, originally from Hornchurch, Essex, England. James had emigrated from Perth, Scotland, "to raise a little flax and a lot of children". Ernest was born at Spring Grove (now Brightwater), near Nelson, New Zealand. His first name was mistakenly spelled Earnest when his birth was registered
He studied at Havelock School and then Nelson College and won a scholarship to study at Canterbury College, University of New Zealand where he was president of the debating society, among other things. After gaining his BA, MA and BSc, and doing two years of research at the forefront of electrical technology, in 1895 Rutherford travelled to England for postgraduate study at the Cavendish Laboratory, University of Cambridge (1895–1898), and he briefly held the world record for the distance over which electromagnetic waves could be detected.
In 1898 Rutherford was appointed to the chair of physics at McGill University in Montreal, Canada, where he did the work that gained him the Nobel Prize in Chemistry in 1908. In 1900 he gained a DSc from the University of New Zealand. Also in 1900 he married Mary Georgina Newton (1876–1945); they had one daughter, Eileen Mary (1901–1930), who married Ralph Fowler. In 1907 Rutherford moved to Britain to take the chair of physics at the University of Manchester
Scientific Research
At the turn of the century, there was little known about atoms except that they contained electrons. J. J. Thompson discovered the electron in 1897, and there was considerable speculation about where these negatively charged particles existed in nature. Matter is electrically neutral; some positive charge must balance the charge of the electron. One popular theory of the time was called the ‘plum pudding model’. This model, invented by Thompson, envisioned matter made of atoms that were spheres of positive charge spiked with electrons throughout. Electrons were chunks of plum distributed through a positively charged sphere of pudding.
In 1911, Ernest Rutherford performed an experiment to test the plum pudding model. He fired energetic a [He2+] particles at a foil, and measured the deflection of the particles as they came out the other side. From this he could deduce information about the structure of the foil. To understand how this works, imagine shooting a rifle at a mound of loose snow: one expects some bullets to emerge from the opposite side with a slight deflection and a bit of energy loss depending on how regularly the pile is packed. One can deduce something about the internal structure of the mound if we know the difference between the initial (before it hits the pile) and final (after it emerges from the pile) trajectories of the bullet. If the mound were made of loose, powdery snow, the bullets would be deflected very little; if the bullets were deflected wildly, we might guess that there was a brick of hard material inside.
Rutherford expected all of the particles to be deflected just a bit as they passed through the plum pudding. He found that most of the a’s he shot at the foil were not deflected at all. They passed through the foil and emerged undisturbed. Occasionally, however, particles were scattered at huge angles. While most of the a’s were undisturbed, a few of them bounced back directly. Imagine if something like this happened at our mound of snow. We shoot bullets at the pile for days, and every round passes straight through, unperturbed – then a bullet hits the snow, reflects back, and splinters the gun’s stock! Rutherford’s result lead him to believe that most of the foil was made of empty space, but had extremely small, dense lumps of matter inside. No other model accounted for the occasional wide angle scattering of the a. With this experiment, Rutherford discovered the nucleus.
Physicists have since used particle scattering in many ways to learn about matter, and have had much success in studying solids. To understand some of the ways that ions are used to probe solids, we consider an important technique used in crystallography: Rutherford Backscattering.
Ernest Rutherford publishes his atomic theory describing the atom as having a central positive nucleus surrounded by negative orbiting electrons. This model suggested that most of the mass of the atom was contained in the small nucleus, and that the rest of the atom was mostly empty space. Rutherford came to this conclusion following the results of his famous gold foil experiment. This experiment involved the firing of radioactive particles through minutely thin metal foils (notably gold) and detecting them using screens coated with zinc sulfide (a scintillator). Rutherford found that although the vast majority of particles passed straight through the foil approximately 1 in 8000 were deflected leading him to his theory that most of the atom was made up of 'empty space'.
members:
Ipac
Jimenez
Lauang
Limpo
J.J. THOMSON II-3
Sir John Joseph "J.J." Thomson
Brief Life Description:
Sir J.J. Thomson is a famous physicist from England. He was born in December 18, 1856 in Cheetham Hill, Mancheter. He attended the Victoria University of Manchester. He dated and later married Rose Elizabeth Paget. He died on 30th of August 1940 in Cambridge. His death was unspecified.
Facts:
-In the year 1897, he tells a startled scientific audience that he's discovered something smaller than an atom. It was a particle with a minuscule mass and a negative charge.
- He was a director of Cavendish Laboratory at Cambridge University.
- Thomson was researching about electrical currents inside Cathode ray tubes.
-Thomson observed that the rays are deflected by an electric field.
Thomson's Conclusion:
He concluded that:
"As the cathode rays carry a charge of negative electricity, are deflected by an electrostatic force as if they were negatively electrified, and are acted on by a magnetic force in just the way in which this force would act on a negatively electrified body moving along the path of these rays, I can see no escape from the conclusion that they are charges of negative electricity carried by particles of matter."
- J.J. THOMSON
A cathode ray. It was used by Thomson in his experiments.
SOURCES
http://people.famouswhy.com/j__j__thomson/
http://en.wikipedia.org/wiki/J._J._Thomson
http://de.academic.ru/pictures/dewiki/74/JJ_Thomson.jpg
group 5
HERRERA- ABSENT
IGNACIOILAO
INOCENCIO
Joseph Louis Proust
Joseph Louis Proust was a french scientist. He was born on September 26, 1754. He developed the use of Hydrogen Sulfide as a reagent. He had the idea that the every pure chemical compound consists of elements of definite proportions. Many chemists of his time disagreed over this. Today, chemists accepted his idea.
Group 10
Danna Santos
Maia Tangco
Milana Tapawan
Diana Yoon
http://en.wikipedia.org/wiki/Joseph_Proust
Group 10
Danna Santos
Maia Tangco
Milana Tapawan
Diana Yoon
http://en.wikipedia.org/wiki/Joseph_Proust
Joseph Louis Proust
Background:
>was born on September 26, 1754 in Angers, France
>he taught Chemistry with a famous astronaut named Pilatre de Rozier in France and taught by himself in Spain
>They burned down his laboratory when Napoleon invaded Spain, so he was forced to return to France
>he died on July 5,1826
Contributions:
He contributed the law of definite proportions. He disapproved Bethrollet.
(He did this by making artificial copper carbonate and comparing it to natural carbonate.)
II-3
Group 10
TANGCO \m/
SANTOSTAPAWAN
YOON
Quantum Mechanics is also known as quantum physics or quantum theory. The Quantum Atomic model is the most recent model of the atom. It's based on quantum mechanics.Quantum numbers are quantum solutions to quantum equations and are used to find energy level an electron is in. The magnetic quantum number indicates that a region within a sub-level called an orbital where two electrons reside. The most common way to describe electrons in atoms according to quantum mechanics is to solve the Schrödinger equation for the energy states of the electrons within the atom. When the electron is in these states, its energy is well-defined but its position is not. The position of an electron is described by a probability distribution map called an orbital.
After Max Planck determined the enery is released and absorbed by atoms in certain fixed amounts known as Quanta, Energy is Quantinized.
After Max Planck determined the enery is released and absorbed by atoms in certain fixed amounts known as Quanta, Energy is Quantinized.
Subscribe to:
Posts (Atom)