Ernest Rutherford
Biography
Ernest Rutherford was the son of James Rutherford, a farmer, and his wife Martha Thompson, originally from Hornchurch, Essex, England. James had emigrated from Perth, Scotland, "to raise a little flax and a lot of children". Ernest was born at Spring Grove (now Brightwater), near Nelson, New Zealand. His first name was mistakenly spelled Earnest when his birth was registered
He studied at Havelock School and then Nelson College and won a scholarship to study at Canterbury College, University of New Zealand where he was president of the debating society, among other things. After gaining his BA, MA and BSc, and doing two years of research at the forefront of electrical technology, in 1895 Rutherford travelled to England for postgraduate study at the Cavendish Laboratory, University of Cambridge (1895–1898), and he briefly held the world record for the distance over which electromagnetic waves could be detected.
In 1898 Rutherford was appointed to the chair of physics at McGill University in Montreal, Canada, where he did the work that gained him the Nobel Prize in Chemistry in 1908. In 1900 he gained a DSc from the University of New Zealand. Also in 1900 he married Mary Georgina Newton (1876–1945); they had one daughter, Eileen Mary (1901–1930), who married Ralph Fowler. In 1907 Rutherford moved to Britain to take the chair of physics at the University of Manchester
Scientific Research
At the turn of the century, there was little known about atoms except that they contained electrons. J. J. Thompson discovered the electron in 1897, and there was considerable speculation about where these negatively charged particles existed in nature. Matter is electrically neutral; some positive charge must balance the charge of the electron. One popular theory of the time was called the ‘plum pudding model’. This model, invented by Thompson, envisioned matter made of atoms that were spheres of positive charge spiked with electrons throughout. Electrons were chunks of plum distributed through a positively charged sphere of pudding.
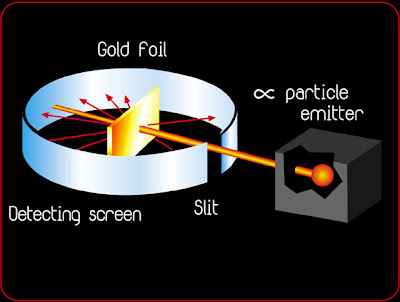
In 1911, Ernest Rutherford performed an experiment to test the plum pudding model. He fired energetic a [He2+] particles at a foil, and measured the deflection of the particles as they came out the other side. From this he could deduce information about the structure of the foil. To understand how this works, imagine shooting a rifle at a mound of loose snow: one expects some bullets to emerge from the opposite side with a slight deflection and a bit of energy loss depending on how regularly the pile is packed. One can deduce something about the internal structure of the mound if we know the difference between the initial (before it hits the pile) and final (after it emerges from the pile) trajectories of the bullet. If the mound were made of loose, powdery snow, the bullets would be deflected very little; if the bullets were deflected wildly, we might guess that there was a brick of hard material inside.
Rutherford expected all of the particles to be deflected just a bit as they passed through the plum pudding. He found that most of the a’s he shot at the foil were not deflected at all. They passed through the foil and emerged undisturbed. Occasionally, however, particles were scattered at huge angles. While most of the a’s were undisturbed, a few of them bounced back directly. Imagine if something like this happened at our mound of snow. We shoot bullets at the pile for days, and every round passes straight through, unperturbed – then a bullet hits the snow, reflects back, and splinters the gun’s stock! Rutherford’s result lead him to believe that most of the foil was made of empty space, but had extremely small, dense lumps of matter inside. No other model accounted for the occasional wide angle scattering of the a. With this experiment, Rutherford discovered the nucleus.
Physicists have since used particle scattering in many ways to learn about matter, and have had much success in studying solids. To understand some of the ways that ions are used to probe solids, we consider an important technique used in crystallography: Rutherford Backscattering.
Ernest Rutherford publishes his atomic theory describing the atom as having a central positive nucleus surrounded by negative orbiting electrons. This model suggested that most of the mass of the atom was contained in the small nucleus, and that the rest of the atom was mostly empty space. Rutherford came to this conclusion following the results of his famous gold foil experiment. This experiment involved the firing of radioactive particles through minutely thin metal foils (notably gold) and detecting them using screens coated with zinc sulfide (a scintillator). Rutherford found that although the vast majority of particles passed straight through the foil approximately 1 in 8000 were deflected leading him to his theory that most of the atom was made up of 'empty space'.
members:
Ipac
Jimenez
Lauang
Limpo



No comments:
Post a Comment